(Dependent & Independent Variables is the fourth and final post in a series on electrolytes and how they influence acid-base chemistry)
From a clinical evaluation standpoint, it’s fairly easy to gain insights into an animal’s acid-base status from a blood sample. A pH measurement gives an estimate of the hydrogen ion concentration, [H+], which can be used along with a CO2 measurement from a blood gas analyzer to calculate the concentration of bicarbonate, [HCO3–], present in the animal’s bloodstream. Even better, these values can be used to develop an effective treatment protocol. It would therefore seem logical that variables such as pH, [H+] and [HCO3–] must be central to a problem and that they are determinant forces in acid-base physiology.
In reality, this assumption is at the core of much of the difficulty and confusion that so often accompanies a developing understanding of acid-base mechanisms. The first three posts in this series demonstrate that acid-base adjustments occur without regard for variables such as bicarbonate. This post will be no different in that regard, and will explore how acid-base variables relate to each other and what that means to acid-base balance.
Dependent & independent variables
An important consideration when evaluating variables and their acid-base relevance is whether changes in their concentration occur independently or whether they depend on changes and interactions among other variables.
Dependent Variables
The concentration of a dependent variable is governed by the concentrations and changes of independent variables. In other words, dependent variables change only as a result of changes in independent ones. Variables such as pH, hydrogen ion concentration [H+] and bicarbonate concentration [HCO3-] are all dependent variables. As such, they cannot cause changes in each other or in independent variables. Their concentrations are simply the results of other factors.
Independent Variables
On the other hand, independent variables are subject to independent variation. Their concentrations are not under the control of other variables. As such, independent variables are amenable to change, and as a result, changes in their concentrations influence acid-base status. This has important implications when attempting to correct an acid-base disturbance. There are three independent variables:
- [SID]: the strong ion difference
- PCO2: the partial pressure of carbon dioxide
- [ATOT]: the total amount of weak acid in plasma
Figure 1 provides a description of each of these independent variables. Feel free to read about PCO2 and [ATOT], but this discussion will focus on [SID] — we are already familiar with strong ions, and adjustments to [SID] are how electrolyte products affect acid-base status. [SID] is simply the sum of all positive strong ions and all negative strong ions. Normal plasma [SID] is around +40.
Independent Variables
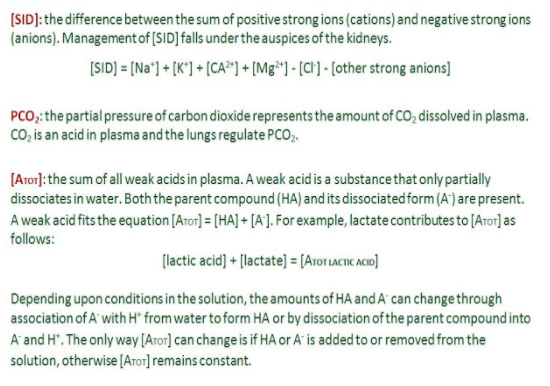 Figure 1.
Figure 1.
Bicarbonate
For the sake of argument, let’s say that it is possible for us to add bicarbonate, a dependent variable, to plasma without the associated sodium. What would happen? The general effect is that the added bicarbonate would travel to the lungs where it would combine with hydrogen to form CO2 and H2O, which would then be exhaled. Each bicarbonate would remove a hydrogen ion from the body. But,since the independent variables have determined the plasma hydrogen ion concentration, we would expect those forces to generate a hydrogen ion to replace each one removed by the added bicarbonate.
But in reality we don’t add bicarbonate by itself — we also add sodium. And sodium concentration is a part of [SID], an independent variable. While bicarbonate is heading off to the lungs to be processed, the added sodium is raising [SID] which lowers the hydrogen ion concentration and raises pH.
So, the notion that bicarbonate is an alkalinizing substance is obviously incorrect. The same can be said for literature that touts bicarbonate’s acid buffering benefits. Nonetheless, sodium bicarbonate is an alkalinizing compound. It is frequently used as a low-cost formulation tool to generate an alkalinizing effect. Formulators often choose not to use sodium bicarbonate because of its potential for causing undesirable effects in the digestive tract. From a formulation standpoint, use of an alkalinizing compound is not required to achieve an alkalinizing effect. Having an alkalinizing compound on the label is no guarantee either. That’s up to the person doing the formulation.
This ends the series on electrolytes and acid-base chemistry. On a personal note, I have always found this subject matter both challenging and fascinating. I hope these discussions have proven interesting and informative, not too painful, and provide useful insights the next time you evaluate an electrolyte product.
______________________________
Other posts in this series:
Jan 18, 2011, Electrolytes – Product Comparisons
Feb 15, 2011, Electrolytes – Dissociation of Strong Ions
May 4, 2011, Electrolytes – Dependent & Independent Variables
